american academy of pediatrics covid vaccine 12-15
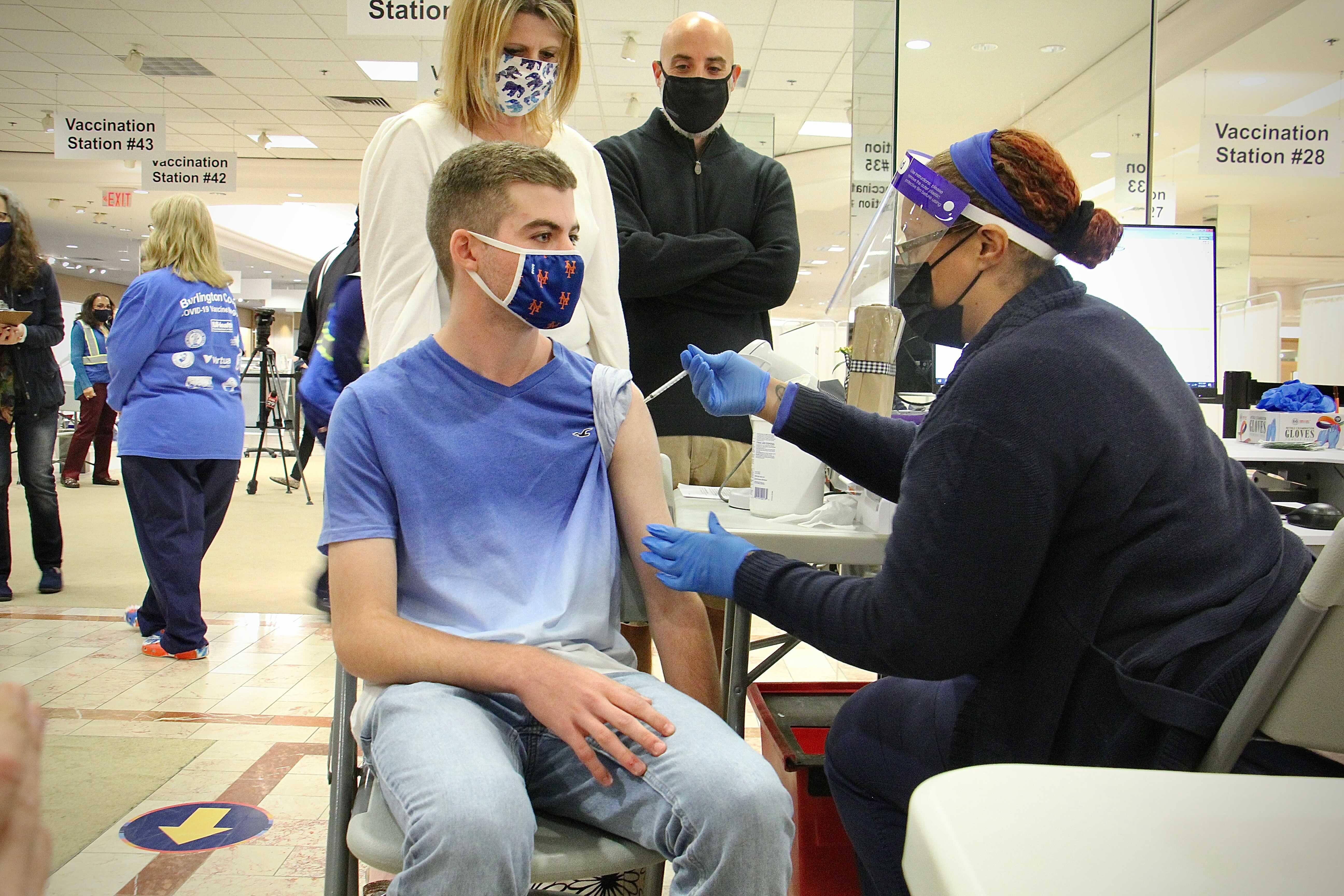
Children accounted for 15 of new Covid cases reported last week American Academy of Pediatrics data analysis shows A 13-year-old shows his bandage after receiving a dose of the Covid vaccine in. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents.

Pfizer Says Its Covid 19 Vaccine Protects Younger Teens Business News Us News
The Pfizer-BioNTech COVID-19 vaccine is now recommended for 12-15 year-olds after the FDA expanded the Emergency Use Authorization EUA to include the adolescent age group for this vaccine.
. The following dosage is the same for everyone 12 and older. The most common side effects in adolescents were pain at the injection site fatigue headache chills muscle pain fever and joint pain consistent with trials in older teens and adults. American Academy of Pediatrics urges families to make COVID vaccines part of their back-to-school checklist.
The Food and Drug Administration FDA has granted emergency use. The AAP recommends COVID-19 vaccination for all children and adolescents 5 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age. The American Academy of Pediatrics and the CDC have independently reviewed the data and the FDAs decision and recommend vaccination for everyone 12 years of age and older.
The Pfizer vaccine has since been fully approved for people 16 and older and remains authorized for emergency use for those 12-15. COVID-19 vaccines are the most important intervention to contain SARS-CoV-2. By Pamela Simms-Mackey MD FAAP.
The AAP recommends against giving the vaccine to children under 12 until authorized by the FDA While the FDA is considering full approval of the vaccine for ages 12 through 15 it is available under emergency use authorization for this age group now and AAP strongly recommends all eligible adolescents be vaccinated as soon as possible. Currently a booster shot is not recommended for children younger than 12 years old. Teens Ages 12 and Older Can Get a Booster Shot.
American Academy of Pediatrics Cautions Against Off-Label Use of COVID-19 Vaccines in Children Under 12. Teens ages 12 to 17 years old should receive the Pfizer-BioNTech COVID-19 booster shot at least 5 months after completing their Pfizer-BioNTech COVID-19 primary series. A booster dose is also strongly encouraged for ages 12 and up who got two doses of the mRNA COVID vaccine at least five months ago.
According to data collected by the American Academy of Pediatrics as of the end of April more than 378 million children have tested positive for COVID-19. The American Academy of Pediatrics has reported that since the start of the pandemic and as of April 2021 385 million children have been infected with COVID-19 303 have died and children are now making up a greater proportion of all COVID-19 infections4 Beyond the physical toll including hospitalizations unpredictable courses with. The best way to slow the emergence of COVID variants is to get vaccinated.
Adolescents 12-15 receive 2 doses of the adult formulation where as children. While questions may now arise about the administration of the vaccine off-label for children aged 11. In a statement the American Academy of Pediatrics recommended COVID-19 vaccination for all children and adolescents 12 years of age and older who do not have a.
COVID-19 Vaccines for Children and Teens. Learn how it safely teaches your childs immune system to recognize and get rid of the coronavirus that causes COVID-19. The American Academy of Pediatrics and the American Academy of Family Physicians recommend vaccinating children aged 5 to 11 years.
Public health officials must build trust. COVID-19 Vaccine for Children Ages 5-11 The American Academy of Pediatrics AAP Advisory Committee on Immunization Practices ACIP Centers for Disease Control and Prevention CDC and the North Dakota Department of Health NDDoH all recommend COVID-19 vaccination of children and adolescents 5 years of age and older. The Centers for Disease Control and Prevention CDC is recommending the Pfizer-BioNTech COVID-19 Vaccine for.
The US Food and Drug Administration is expected to grant emergency use authorization next week to PfizerBioNTechs coronavirus vaccine for teens and children ages 12 to. MRNA COVID-19 vaccines are FDA-approved or authorized for a 3-week Pfizer-BioNTech vaccine or 4-week Moderna vaccine interval between the first and second dose. Currently the Pfizer-BioNTech mRNA vaccine has been authorizedrecommended for children and adolescents aged 5-15.
On December 11 2020 the US Food and Drug Administration FDA issued an Emergency Use Authorization EUA for the BNT162b2 COVID-19 vaccine Pfizer-BioNTech for individuals aged 16 years or older 7 which was expanded to include children aged 12 to 15 years or older on May 10. The American Academy of Pediatrics AAP recommends the COVID-19 vaccine for ages 5 and older. Pfizer and BioNTech conducted trials in more than 2000 adolescents ages 12-15 with half randomized to receive the vaccine and half to receive a placebo.
ITASCA IL -- Today the Food and Drug Administration FDA finalized the full approval of the Pfizer-BioNTech COVID-19 vaccine for ages 16 and older. More than 60 of. If your teen or child is healthy but has not had their COVID vaccine dont wait.
A 3- or 4-week interval continues to be the recommended interval for people who are moderately to severely immunocompromised children who are 5-11 years old and others who need rapid protection. The American Academy of Pediatrics AAP Advisory Committee on Immunization Practices ACIP Centers for Disease Control and Prevention CDC and the North Dakota Department of Health NDDoH all recommend COVID-19 vaccination of adolescents ages 12 years and older. 910 Yet there are large divides in public trust especially for pediatric COVID-19 vaccines.
The Pfizer vaccine became available in the United States in December 2020 for people 16 or older for 12- to 15-year-olds in May 2021 and for 5- to 11-year-olds in October 2021. 8 This was based on a randomized double-blind placebo-controlled. Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible.
Especially as children now make up 15 of COVID-19 cases nationwide Dr. The Autism Society of America encourages parents to consult their childs physician when determining if the COVID-19 vaccine is recommended for their eligible child. The American Academy of Pediatrics answers questions parents may have about the COVID-19 vaccine.
American Academy of Pediatrics urges FDA to approve COVID vaccines for children under 12 Sep 13 2021 640 PM EDT.
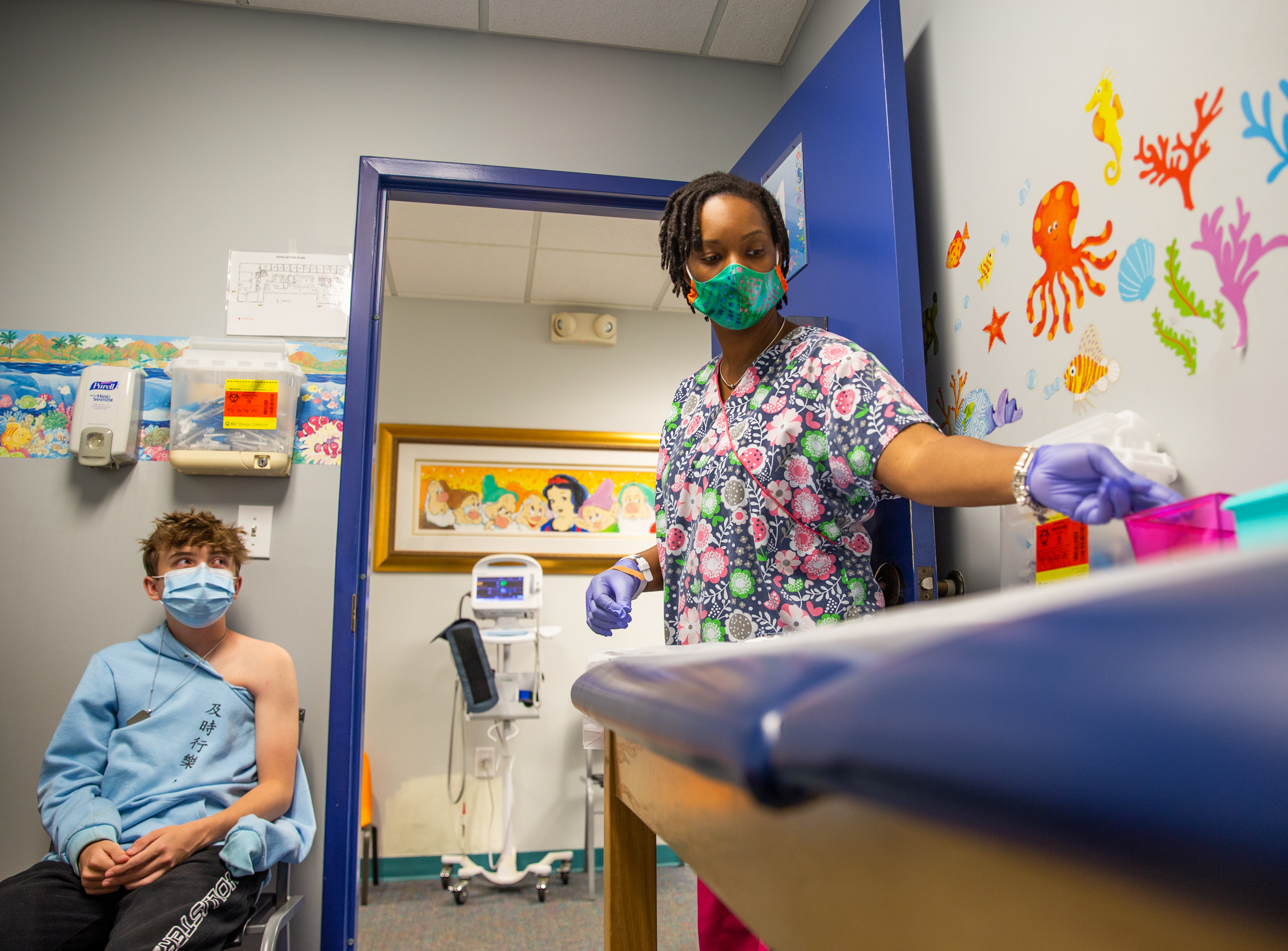
Fda Authorizes Pfizer Covid Vaccine For Children 12 To 15

Faq Covid 19 Vaccine For Children Phoenix Children S Hospital

What Do We Know About Covid 19 In Kids Now Whyy
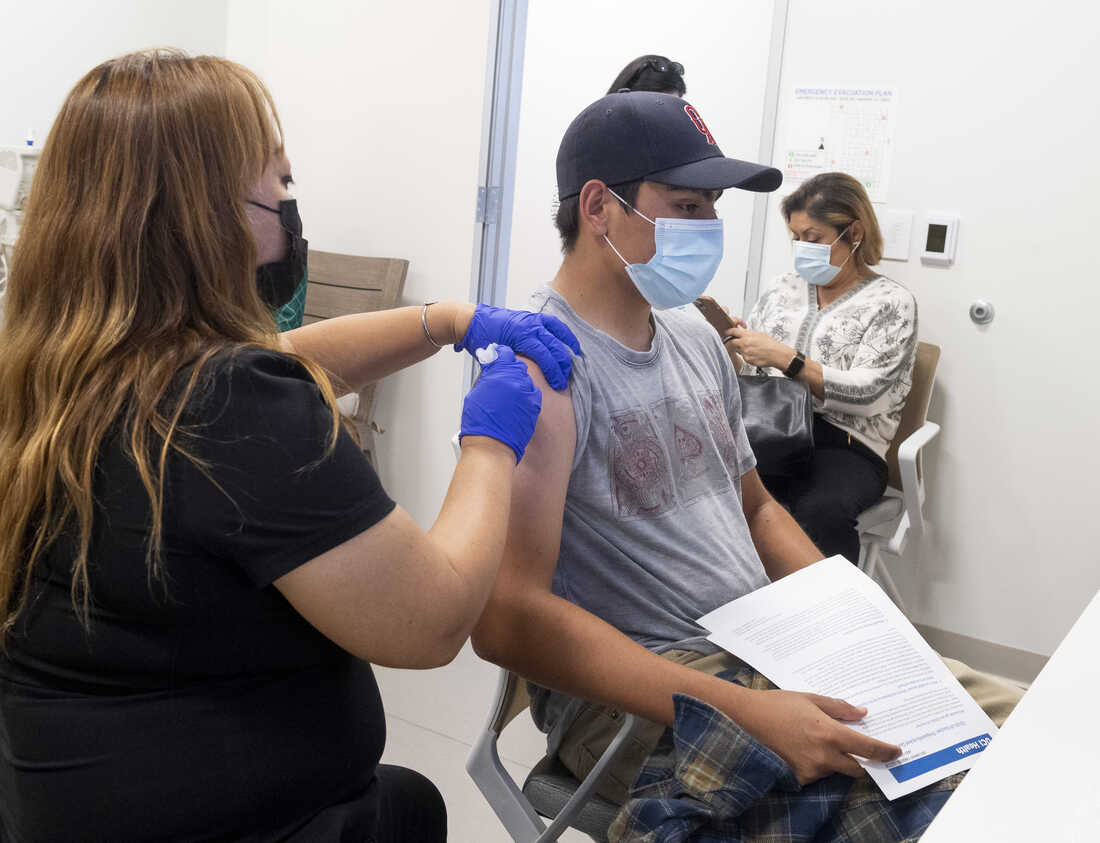
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says

Aap Recommends Covid 19 Vaccine For Ages 16 And Up

Fact Check How Useful Are Coronavirus Vaccines For Children And Adolescents Coronavirus And Covid 19 Latest News About Covid 19 Dw 06 08 2021

Covid 19 Vaccines On Your Back To School Checklist Extension Monroe County

Children As Young As 12 May Soon Be Able To Get Vaccinated

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News

Covid 19 Vaccine For Kids Benefits Are Many Here Are Answers To Parental Concerns Ask The Pediatrician Column Together Lancasteronline Com
Younger Kids Could Be Getting A Covid 19 Vaccine Within Weeks Cnn
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org

Covid 19 Vaccine For 12 To 15 Year Olds What Parents Should Know Methodist Health System Omaha Council Bluffs Fremont

Kids Covid Vaccine Twitter Chat Twitter

As School Resumes Uf Health Pediatrics Expert Gives Advice On Covid 19 Safety Uf Health University Of Florida Health
Kids Covid Vaccine Side Effects What To Know

Cdc Panel Recommends Pfizer Biontech Vaccine For Kids 12 To 15 Years Old

Cdc Committee Recommends That 12 To 15 Year Olds Receive The Pfizer Biontech Covid Vaccine